Abstract
Multiple myeloma is a largely incurable bone marrow (BM) resident plasma cell malignancy that is increasing in incidence. Autologous stem cell transplantation (ASCT) is the current standard consolidation therapy and a subset of patients achieve durable progression free survival that is suggestive of long-term immune control. Utilizing novel preclinical models, we have provided definitive evidence that this is largely mediated by T cell-dependent myeloma-specific immunity. In both patients and preclinical models, myeloma progression is associated with T cell dysfunction and expression of multiple inhibitory receptors suggesting a loss of immunosurveillance. In mice, we have demonstrated potent anti-myeloma efficacy of TIGIT blockade in both ASCT and non-transplant settings. Here we utilized identical TIGIT Abs that do or do not Fc bind to demonstrate that immunological efficacy after ASCT was absolutely dependent on ADCC (median survival was unreached (>110 days) in Fc-binding vs 73 days in Fc-dead and 71 days in control Ig (cIg)-treated mice). Since TIGIT inhibition does not protect against myeloma relapse in all mice, it is apparent that combinational approaches are required to target non-responders. Therefore, we hypothesized that TIGIT blockade could be combined with immunomodulatory drugs (IMiDs) to provide synergistic anti-myeloma activity after ASCT.
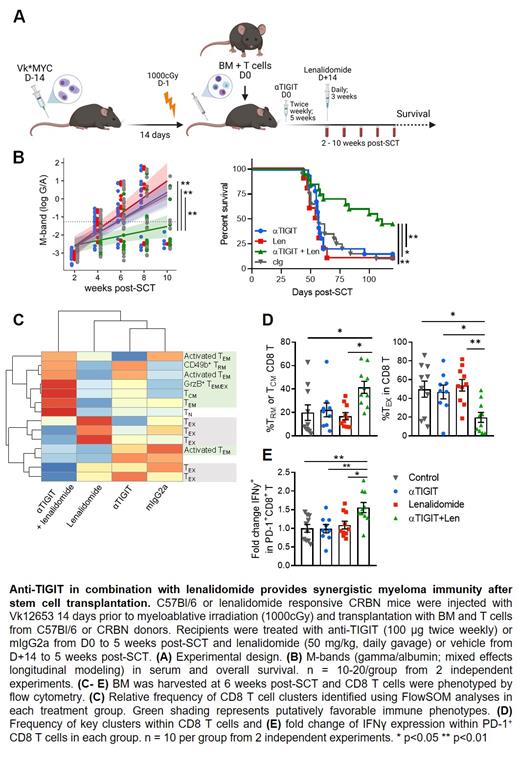
To that end, we utilized CRBN transgenic mice to investigate the efficacy of TIGIT blockade in combination with lenalidomide, the standard of care IMiD used in maintenance therapy after clinical ASCT. Briefly, B6 Vk*MYC myeloma-bearing (MM-bearing) mice were lethally irradiated and transplanted with B6 bone marrow (BM) and a suboptimal dose of T cells followed by anti-TIGIT or control Ig (100 mg twice weekly) for 5 weeks with lenalidomide (50 mg/kg daily gavage) or control diluent from D+14 for 3 weeks (Figure 1A). The combination of anti-TIGIT and lenalidomide provided synergistic anti-myeloma efficacy evidenced by prolonged median survival (109 days in combination vs < 60 days in monotherapy/control-treated mice, p<0.01; Figure B). Myeloma M bands were also suppressed in the combination treated mice relative to monotherapy or cIg-treated mice (p<0.01; Figure 1). Analysis of BM CD8 T cells 6 weeks after ASCT demonstrated that combination therapy significantly decreased terminal exhaustion (TOX + TIM3 + CD101 + PD-1 + DNAM-1 ─) with an average of only 20% of CD8 T cells with an exhausted phenotype in the combination group compared to greater than 50% exhausted CD8 T cells in monotherapy or cIg-treated mice (p<0.05; Figure 1C-D). The combination also increased the frequency of central memory and tissue-resident memory subsets (CD49b +CD69 +; p<0.05; Figure 1C-D), and increased IFNγ production from activated (PD-1 +; p<0.05; Figure 1E) cells compared to monotherapy or control Ig-treated mice. Importantly, these phenotypic changes were specific to the BM tumor microenvironment as we observed no effect of combination or monotherapy treatment on CD8 or CD4 T cells in peripheral blood.
In sum, these data provide a strong rationale for combining TIGIT inhibition with immunomodulatory drugs to prevent the progression of myeloma.
Driessens: iTeos Therapeutics: Current Employment, Current equity holder in publicly-traded company. Holmberg: Up-To-Date: Patents & Royalties; Bristol Myers Squibb: Research Funding; Janssen: Research Funding; Merck: Research Funding; Millennium-Takeda: Research Funding; Sanofi: Research Funding; Seattle Genetics: Research Funding. Hill: NeoLeukin Therapeutics: Consultancy; Compass Therapeutics: Research Funding; NapaJen Pharma: Consultancy; Generon Corporation: Consultancy; Roche: Research Funding; iTeos Therapeutics: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding; Applied Molecular Transport: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal